Units and Dimensions Short Notes and Formulae (Part-2)– Physics Basic facts and fundamentals for Board exam and IIT JEE NEET NDA other Competitive Exams
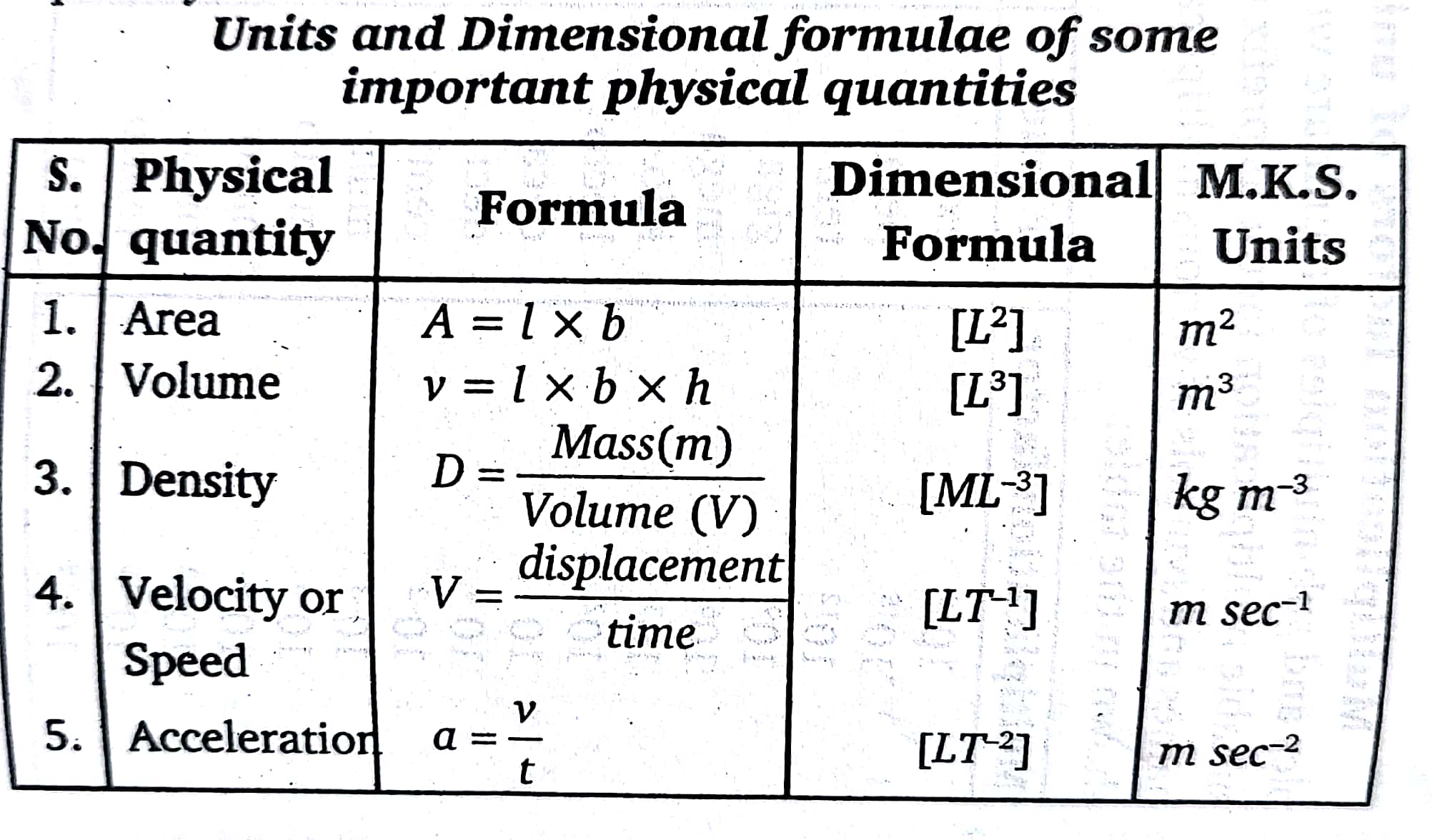
Some Physical Quantities having Same Dimensions
1.Momentum and Impulse : [MLT-1]
2. Work, Energy, Moment of force, Torque :[ML2L-2]
3. Pressure, Stress, Modulus of Elasticity: [ML-1 T-2]
4. Plank’s constant, Angular momentum : [ML2 T-1]
5. Surface Tension, Force constant : [MT-2 L0]
6.Frequency, Decay constant, Angular velocity, Velocity gradient : [M0 L°T-1]
7.Gravitational Potential, Latent heat : [M0 L2 T-2]
8.Thermal capacity, Centropy : [ML2 T-2 K-¹]
Some Important Units of Length:
1 micron (1μ) = 10-4 cm = 10-6 m
1 Angstrom (1A°) = 10-8 cm = 10-10m
1 Fermi (1F) = 10-13 cm = 10-15m
1 x U= 10-11 cm = 10-13m
Light year = Distance travelled by light in vacuum in one year = 9.46 x 1015m ⁓1016m.
1 Parse = 3.08 x 1016m = 3.26 light years.
Astronomical unit- Average distance between sun and earth of radius of earth’s orbit. It is used in astronomy to measure distance of planets.
1 AV 1.496 x 1011m = 1.496 x 108 km.
Some practical units of the standard of mass-
(i) Chandra Shekhar Limit (C.S.L.)
It is the largest practical unit of mass.
1 C.S.L= 1.4 times the mass of sun.
(ii) 1 Metric ton = 100 kg
(iii) Atomic mass unit (a.m.u.)-
It is not the atomic standard of mass, but a practical unit of mass used in atomic and nuclear physics and is the smallest unit. It is defined at present as 1/12 of the mass of one C¹² atom.
1 a.m.u. = 1/12 of mass of C12 atom 12
= 1.67 x 10-27 kg.
Some practical units of standards of time
(i) Century-It is the largest unit of time.
(ii) Year-It is the time taken by earth to complete one rotation around the earth in its orbit.
(iii) Lunar month-It is the time taken by moon to complete one rotation around the earth in its orbit.
1 L.M. = 27.3 days.
(iv) Solar day-It is the time taken by the earth to complete only one rotation about its axis with Mit respect to sun.
(v) Mean solar day-Average of solar days taken over one solar year is called mean solar day. 1 solar year = 365.25 mean solar days. Since 1 year = 365 days, there occur a difference of 1 day every fourth year.
(vi) Siderial day-It is the time taken by earth to complete one rotation about its axis with respect to a distant year.
(vii) 1 Shake-Smallest and obsolete unit of time.
1 shake =10-8 sec.
Uses of Dimentional Equations-
(a) To check the validity of a given equation or formula-To check the validity of a given formula– we keep the dimensions of different physical quantities on both sides of the equation. If the powers of M, L & T on L.H.S. of the equation are equal to the corresponding powers of M, L and T respectively on the R.H.S. of the equation, then the formula is correct. If they are not equal, then the formula is incorrect.
(b) To derive the formula for given physical quantity-If the different items on which a physical quantity depends to known then the equation of that physical quantity can be obtained on the principal of homogeneity.
(c) To convert one system of physical units into another-To convert one system of units into another, let us consider that n, and n, are the numerical values of the same quantity in two different systems and [MLT] and [M, LT2] are the dimensional formulae in these systems. Then,
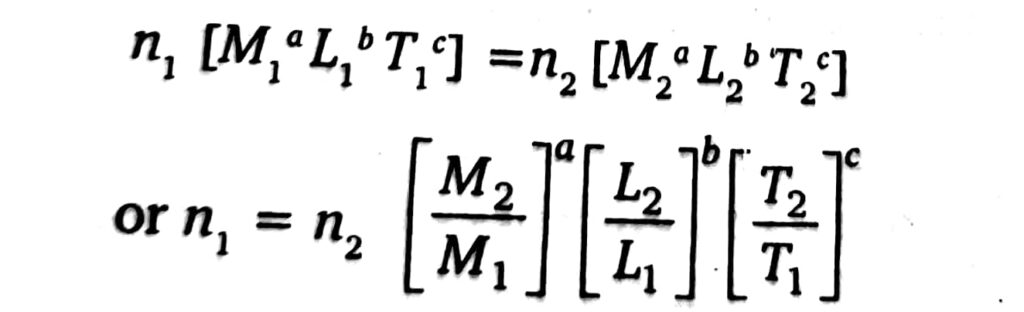
Principle of Homogeneity-The dimensions of both sides in an equation are same.
For example-
S = ut + (1 /2)gt²
L= [LT-1 . T] + [LT-2 . T2]
or L = [L] + [L]
Defects of Dimensional Analysis-
(i) While deriving a formula the proportionality constant cannot be found.
(ii) The formula for a physical quantity depending os on more than three other physical quantities cannot be derived. It can be checked only.
(iii) The equations of the type v = (u ± at) can not be derived. They can be checked only.
(iv) The equations containing trigonometrical functions (sin ɵ, cos ɵ, etc.), logarithmic functions (log x, log x3 etc.) and exponential functions (ex, ex2 etc.) can not be derived. They can be checked only.
(v) 1/√µ0ɛ0 has the dimensions of velocity. It is equal to velocity of light in vaccum.
(vi) √(μ_ε/(μ_0 ε_0 )) has no dimensions. It equals refractive index ‘u’.
(vii) Plane angle, solid angle; coefficient of friction strain, refractive index, Paission’s ratio etc. have no dimensions.
(viii) Dimension less quantity has same value in all system of units e.g. strain, coefficient of friction, refractive index etc.
(ix) Plane angle and solid angle have units but no dimensions.
Order of Magnitudes-
Some times the abso- lute value of the physical quantity is not needed, only the order of magnitudes of the physical quantity are required. To know the order of magnitude of a number, we adopt the following rule:
(i) “If the number is less than 5, then it a 1, if it is between 5 and 10, take it as 10.” Examples-
(a) Velocity of light is 3× 108 m/sec, therefore, its order of magnitude is 108 m/sec.
(b) Electronic charge is 1.6 x 10-19 coulomb. Therefore, its order of magnitude of the electronic charge is 10-19 coulomb.
(c) The number of minutes in one hour is 60 = 6x 10, therefore, its order of magnitude is 102.
Errors in Measurement
The measured value of a physical quantity is usually different from its true value. The result of every measurement by any measuring instrument is an approxi- mate number, which contains some uncertainty. This uncertainty is called error.
(a) Classified Errors-These errors are pro- duced due to the structure of instrument, mechanism of measurement, variation of physical quantity during the measurement or due to personal errors. They may be following-
Order of time interval(s)
10-15
10-22
Time taken by light to cross distance of Nuclear size
hydrogen atom
Time period of electron in